Chemistry might sound boring or confusing at first, especially when we see complex formulas like hcooch ch2 h2o. But don’t worry! You don’t need to be a scientist to understand what these letters and numbers mean. This article will help you learn everything you need to know about this chemical combination in a simple, friendly, and fun way.
Whether you’re a student, a curious learner, or just want to know what these formulas are, we’re here to explain it all. We’ll break down the basics of chemistry, how HCOOCH reacts with CH₂ and H₂O, and why this matters in real life. You’ll also learn how water plays a role and how chemical reactions like this are used in everyday things like cleaning products, fuels, and even in nature.
Let’s explore this cool world of atoms and molecules, one step at a time.
What Does HCOOCH Mean?
First, let’s talk about the formula HCOOCH. This is a shorthand version of a compound called methyl formate. It’s made up of hydrogen, carbon, and oxygen atoms. The full name looks more like HCOOCH₃, which stands for a formic acid ester (methyl ester of formic acid).
Here’s what each part means:
- HCOO- is the formate part, which comes from formic acid.
- CH₃ is the methyl group, a common part of organic compounds.
When you put them together, you get methyl formate.
This compound is a colorless liquid with a pleasant smell, kind of like rum. It’s used in things like perfumes, solvents, and insecticides. Sometimes, it’s even a byproduct in chemical industries. Scientists like it because it’s simple, but it’s also very useful for research and experiments.
What Is CH₂ in This Reaction?
The CH₂ part in HCOOCH CH₂ H₂O likely refers to a methylene group, which contains one carbon and two hydrogen atoms. This group is highly reactive and often found in many organic compounds.
CH₂ can come from molecules called alkenes, like ethene (C₂H₄). It plays a big role in reactions where molecules want to join together, like in polymer chemistry or esterification. In this case, CH₂ can act like a “bridge” or connector between other atoms, helping the formate do its job when water (H₂O) is also involved.
Even though it looks simple, CH₂ is a very important building block in chemistry. Without it, lots of chemical products would not exist!
Why Is Water (H₂O) So Important in Chemistry?
We all know what H₂O, or water, is. We drink it, bathe in it, and use it to grow plants. But in chemistry, water can also act like a reaction helper. Sometimes, water helps break molecules apart or helps them come together—even change them entirely.
In reactions where HCOOCH CH₂ H₂O are together, water usually acts as a solvent (a space where reactions can happen) or a reactant (a part of the chemical reaction). One common process involving water is hydrolysis, which means breaking down big molecules into smaller ones using water.
Water is also neutral, which means it’s very gentle and useful for reactions that need a balanced setup. That’s why water often appears in chemical formulas and everyday chemical reactions.
How Do These Three Work Together: HCOOCH, CH₂, and H₂O?
Now let’s connect the dots! The chemical combination HCOOCH CH₂ H₂O can be understood as a type of organic chemical reaction involving:
- Methyl formate (HCOOCH)
- A methylene group (CH₂)
- Water (H₂O)
One possible reaction between these elements is a hydration or hydrolysis reaction. Depending on the catalyst (something that speeds up the reaction), these components can create different products.
For example, in a lab:
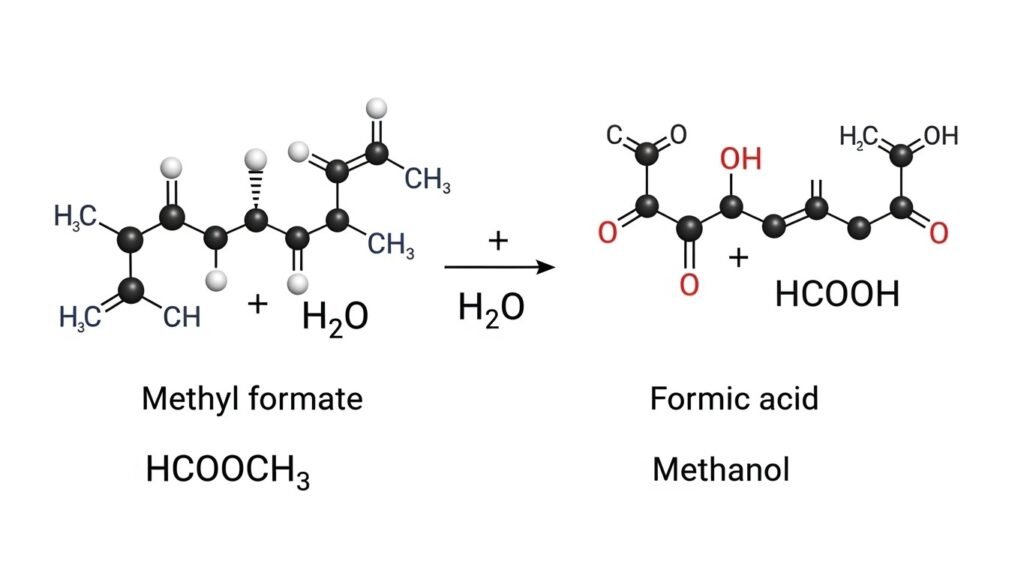
- HCOOCH (methyl formate) can react with water to form formic acid and methanol.
- CH₂ may act as a linking piece or appear as part of another compound that brings these together.
The real importance of this reaction lies in how it mimics what happens in both industrial processes and biological systems.
A Real-Life Example: Methyl Formate in Industry
You’ve probably never seen a bottle labeled “methyl formate” under your kitchen sink, but this chemical is quietly everywhere. It’s used in:
- Making foams for furniture cushions
- Paint and coating industries
- As a blowing agent (to create air pockets inside materials)
- To produce other chemicals like formic acid
When methyl formate reacts with water (our H₂O) in these applications, it changes form and helps create new substances. The small CH₂ groups are everywhere in this process—they’re the skeleton structure of thousands of chemical reactions that create all kinds of materials.
So while HCOOCH CH₂ H₂O might look like just some boring formula, it’s at the heart of real, useful products in our everyday world.
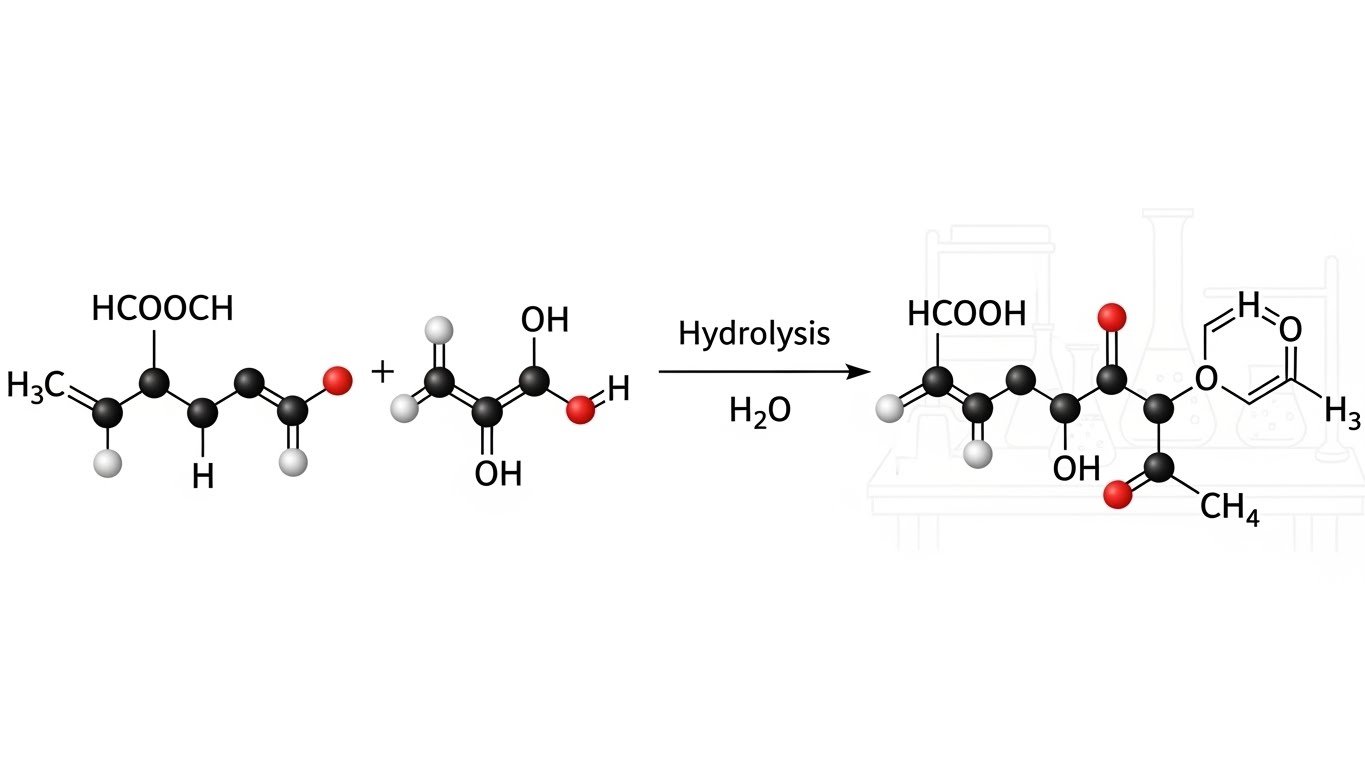
The Science Behind Hydrolysis: Breaking Molecules With Water
Let’s talk more about hydrolysis, one of the key ideas behind the mix of HCOOCH CH₂ H₂O. Hydrolysis happens when water is used to split a chemical bond.
In this case:
- HCOOCH could break into formic acid (HCOOH) and methanol (CH₃OH) after reacting with H₂O.
This is a classic ester hydrolysis reaction.
Hydrolysis is important in nature too. It helps break down:
- Food into energy in our stomachs
- DNA and proteins in cells
- Big molecules in the environment
It’s a soft but powerful way nature and science change molecules into useful forms.
What Are Esters and Why Do They Matter?
fact, methyl formate (HCOOCH) is a type of ester.
Esters are made when acids react with alcohols. They often smell sweet or fruity. That’s why esters are used in:
- Perfumes
- Candies and flavorings
- Air fresheners
Esters can also be broken down with water (thanks to H₂O) in a reaction called—you guessed it—hydrolysis. Knowing about esters helps you understand why HCOOCH CH₂ H₂O is so useful in both science and daily life.
A Closer Look at Methyl Formate’s Structure
Let’s go deeper into the structure of methyl formate (HCOOCH).
Here’s a very basic diagram idea:
- HCOO is the formic acid part
- CH₃ is the methyl part
They are connected through an ester bond, which can be broken by water.
The CH₂ group may represent a part of another molecule reacting with methyl formate or may be shown as part of a different process where CH₂ gets added or removed. These tiny molecular groups play big roles in how the whole chemical behaves!
Environmental Benefits and Concerns
While HCOOCH and its reactions are useful, it’s important to think about safety too. Methyl formate is flammable and can cause irritation if breathed in or touched too much.
Good news though: it breaks down quickly in the environment and doesn’t build up in water or soil. That’s one reason it’s considered better than some other industrial chemicals.
Still, chemists handle it carefully, making sure it doesn’t escape into the air in large amounts. Using water (H₂O) and other safe chemicals in reactions with HCOOCH helps reduce harmful effects.
How Are These Chemicals Used in Experiments?
In chemistry labs, HCOOCH CH₂ H₂O combinations are often used to study:
- Reaction speed
- Molecule stability
- How esters break down into acids and alcohols
- Creation of new organic compounds
Simple reactions like this are often a part of introductory organic chemistry classes, helping students learn how real chemists build and break molecules.
Working with safe amounts and the right tools, students and scientists can explore how small changes in molecules lead to different products. It’s like playing with LEGO bricks—but on a molecular level!
How Can Students or Beginners Explore This?
If you’re learning chemistry and want to explore what HCOOCH CH₂ H₂O means, start by understanding:
- Simple formulas (like H₂O = water)
- How carbon (C), hydrogen (H), and oxygen (O) form molecules
- What esters are and how they react with water
- How atoms bond to make bigger molecules
You don’t need to memorize everything. Just start with curiosity. Use chemistry kits, watch fun science videos, or join a school science fair. Learning about HCOOCH CH₂ H₂O is a great entry into understanding how chemistry helps build the world around us.
FAQs
1. What does HCOOCH stand for?
It stands for methyl formate, a simple organic chemical made from formic acid and methanol.
2. What role does water (H₂O) play in this formula?
Water often helps create or break chemical bonds in reactions like hydrolysis, helping turn esters into acids and alcohols.
3. Is HCOOCH dangerous?
In small amounts and controlled settings, it’s safe to use. It is flammable and can be harmful if inhaled in large quantities.
4. What is the CH₂ group in this reaction?
CH₂ is a reactive group of one carbon and two hydrogen atoms, often acting as a connection point in chemical reactions.
5. Where is this chemical used in real life?
You’ll find HCOOCH in perfumes, insecticides, foam manufacturing, and other industrial applications.
6. Can students learn from reactions like HCOOCH CH₂ H₂O?
Absolutely! It’s a great way to understand real-life organic chemistry, esters, and how water affects molecules.
Final Thoughts
At first glance, HCOOCH CH₂ H₂O might just look like a complicated jumble of letters and numbers. But once you understand what each part means, it opens up a whole world of science, creativity, and chemistry wonder.
This simple reaction teaches how real products are made, how nature breaks down waste, and how water itself plays a powerful role in shaping the materials of our lives. Whether in a cleaning product, a bottle of perfume, or a school science lab—it all starts with understanding these clever combinations of atoms.
Stay curious, stay safe, and remember: chemistry isn’t just about formulas—it’s about how those formulas help build our world.