As the world moves rapidly towards electrification, advanced high-performance batteries are essential for powering electric vehicles, consumer devices, and grid storage. Lithium-ion batteries have become the dominant battery technology for consumers in electronics and electric vehicles nowadays.
However, there are concerns about the limited availability and high cost of lithium resources. This has driven research into alternative battery chemistries that could outperform lithium-ion batteries.
One extremely promising new battery type is the fluoride ion battery. Fluoride Ion Battery has 10 times higher energy density than currently available Lithium-ion batteries.
Now, let’s discuss the new battery technology and the science of fluoride ion battery.
How Do Fluoride Ion Battery Works?

Like all batteries, a fluoride ion battery consists of two electrodes – a positive cathode and a negative anode – immersed in an electrolyte solution. During discharge, fluoride ions (F-) flow from the anode to the cathode through the electrolyte, generating an electric current. This is coupled with a reverse reaction during charging where the fluoride ions flow back to recharge the battery.
Fluoride ion battery science :
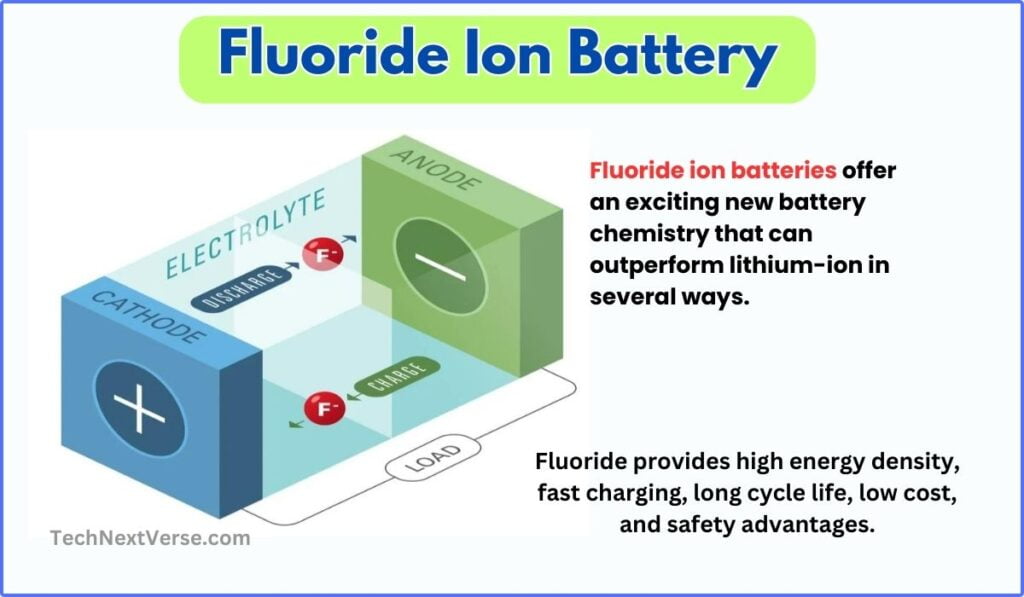
▶ Cathode :
The cathode in fluoride ion batteries is typically made from a metal fluoride, such as lanthanum fluoride (LaF3) or sodium fluoride (NaF). The anode uses an intercalation material such as graphite or titanium dioxide(TiO2 – “a white inorganic compound“) to host fluoride ions. The electrolyte is a solvent containing dissociated fluoride salts. The metal atom has a highly electronegative fluoride ion bonded to it, giving the cathode a high energy density.
▶ Anode :
The anode is made from an intercalation material such as graphite or titanium dioxide. These materials have layered structures with spaces in between that can host fluoride ions.
When discharging, the fluoride ions intercalate into spaces in the graphite or titanium dioxide anode, while the metal atoms in the cathode get reduced. The ions travel back during recharging. The reactions are highly reversible, enabling efficient battery operation.
The electrolyte is a solvent containing fluoride salts. When dissolved, these salts dissociate into free fluoride anions (F-) and cations. Common fluoride salts used include sodium fluoride (NaF), potassium fluoride (KF) and magnesium fluoride (MgF2).
Beyond Lithium-Ion: How Solid State Battery Tech Could Transform Energy Storage

During discharge:
- The fluoride anions in the electrolyte migrate and intercalate into the layered structure of the anode material.
- At the cathode, the metal atom (e.g. La in LaF3) is reduced by gaining electrons from the external circuit.
- This reduction reaction frees up F- ions from the cathode into the electrolyte to maintain charge neutrality.
- The overall result is fluoride ions moving from cathode to anode, generating a flow of electrons in the external circuit i.e. electric current.

During charging, the reverse process occurs:
- Fluoride anions de-intercalate from the anode and migrate back to the cathode through the electrolyte.
- At the cathode, the reduced metal atoms (La in the case of LaF3) get re-oxidized to their original state by losing electrons.
- To maintain charge neutrality, fluoride ions get discharged from the electrolyte and bond back with the oxidized metal atoms.
- This restores both electrodes to their original discharged states, completing the charging cycle.
The high electronegativity of fluoride enables high-voltage cathodes. Its small size allows rapid diffusion through the electrolyte and intercalation into anodes, enabling fast charge-discharge. The process is highly reversible, allowing efficient cycling. Overall, these reactions enable high energy density, power density and cycle life for fluoride ion batteries.

Advantages Of Lithium-ion Batteries
Fluoride ion battery offers several potential advantages compared to lithium-ion batteries:
- Higher energy density – Fluoride can enable up to 5-10 times higher energy density versus lithium-ion. This means more energy storage in a smaller battery.
- Faster charging – The swift fluoride ion diffusion enables faster charge and discharge. Full charging can occur in 10-20 minutes.
- Better stability – Fluoride shuttles between the electrodes exhibit less dendrite formation than lithium. This improves battery lifetime and reduces safety risks.
- Low cost – Fluorine is abundantly available in mineral deposits and salts. Fluoride materials are relatively inexpensive.
- Environmentally friendly – Fluoride ions are stable and non-toxic. The batteries avoid the use of hazardous or scarce elements like cobalt.
These advantages make fluoride ion batteries well-suited for electric vehicles, consumer electronics, and grid-level storage applications. Their high power and enhanced safety can enable new use cases not practical with lithium-ion.

Ongoing Research and Development
Fluoride ion batteries were first demonstrated in laboratories in 2016. Since then, significant research has focused on improving various components:
- Cathodes – Various metal fluorides like YF3, BiF3 and FeF3 are being evaluated to optimize voltage and capacity. Doping with ions like sulfur or selenium has been shown to improve cathode conductivity and stability.
- Anodes – In addition to graphite and titanium dioxide, materials like nanodiamonds and copper fluoride show promise to host high densities of fluoride ions. 3D porous morphologies further boost ion storage capacity.
- Electrolytes – Researchers are developing new classes of liquid electrolytes with dissociated fluoride salts, high ionic conductivity and wide electrochemical windows. Solid-state electrolytes are also being explored.
- Binders & interfaces – Work is ongoing to optimize binders and interfaces to enable high-voltage stability along with minimized undesirable side reactions.
- Battery engineering – Improved battery engineering and designs for fluoride ion batteries are being researched to enable better thermal control, safety, and high-power capabilities.
startups like Alumina Energy and FIBattery Technologies are at the forefront of commercializing this technology. With continued research advances, fluoride-ion batteries could start displacing lithium-ion batteries within the next 5-10 years.

Conclusion
In summary, fluoride ion batteries offer an exciting new battery chemistry that can outperform lithium-ion in several ways. Fluoride provides high energy density, fast charging, long cycle life, low cost, and safety advantages. Ongoing R&D is rapidly improving fluoride battery components and systems.
Commercial viability is on the horizon, with the potential to unlock new applications in electric vehicles, grid storage and consumer electronics. Fluoride ion batteries’ position could emerge as vital complements and eventual successors to lithium-ion batteries over the coming decade.
FAQs
Q: How is fluoride obtained for use in batteries?
A: Fluoride used in batteries is sourced from abundant mineral deposits like fluorspar and produced synthetically from salts. It is widely available and inexpensive.
Q: Is fluoride safe to use in batteries?
A: Fluoride is non-toxic and chemically stable, making it very safe for battery systems compared to elements like cobalt used in lithium-ion batteries. Strict safety standards are followed in battery manufacturing.
Q: How easy is it to recycle fluoride-ion battery?
A: Fluoride battery materials can be easily recovered and recycled at end of life. The recyclability and environmental safety of fluoride gives it an advantage over lithium-ion.
Q: Can fluoride ion batteries explode or catch fire?
A: Fluoride ion batteries exhibit very high thermal and chemical stability. The chances of fire or explosion are extremely minimal compared to some lithium-ion batteries.
Q: Are fluoride batteries compatible with existing chargers?
A: Fluoride batteries require specialized chargers designed for faster and more efficient charging. Existing chargers are incompatible.
Q: What is the lifespan of a fluoride ion battery?
A: Fluoride batteries can deliver over 10,000 charge/discharge cycles with negligible capacity loss. This gives them 3-4 times longer lifespan compared to typical lithium-ion batteries.
Q: Are fluoride ion batteries already being used commercially?
A: Fluoride batteries are currently in the research and testing stages. Widespread commercialization in electric vehicles and other markets is expected in the next 5-10 years.









